Abstract
INTRODUCTION: Acute myeloid leukemia (AML) is a hematological malignancy that relapses frequently, even if remission is achieved with intensive chemotherapies. Therefore, the eradication of residual leukemia cells that survive attack of cytotoxic agents is necessary for robust treatment, and tumor immunity is attracting attention as an alternative modality. In recent years, novel treatments that utilize anti-tumor immunity have been successfully applied for the treatment of lymphoid malignancies and conferred improved outcomes. However, effective immunotherapy for AML remains elusive owing to the lack of optimal target antigens. The nectin-related pathway is of interest as a checkpoint molecule. DNAM-1 (DNAX accessory molecule-1, CD226) and TIGIT (T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain) are expressed on T cells and NK cells, and constitute activating and inhibitory immunoreceptors of nectin-related pathway. These receptors share ligands CD155 (poliovirus receptor, PVR) and CD112 (nectin-2), expressed on tumor cells. Thus, we aimed to develop a novel NK cell therapy by engineering nectin-related immunoreceptors and analyzed their anti-AML activity.
METHODS: Using a public database (http://www.genomicscape.com/), we analyzed the association between CD155 and CD112 expression and prognosis in patients with AML. The expression of these ligands in myeloid leukemia cell lines (MOLM-13, MV-4-11, THP-1, KG-1, and K562) was analyzed using flow cytometry (FACS). Primary samples from patients with de novo AML were collected to analyze the expression of nectin-related pathway molecules, and their association with the outcome of chemotherapy. NK-92 DNAM-1 cells were generated by transfection with the active receptor DNAM-1 using a lentiviral vector. NK-92 TIGIT KO cells were generated and knocked out (KO) inhibitory receptor TIGIT by gene editing using CRISPR-Cas9. The direct cytotoxicity of these modified NK-92 cells against myeloid leukemia cell lines was analyzed. RNA sequencing was performed on the generated NK-92 cells and wild-type (WT) NK-92 cells to analyze and extract genes with enhanced expression. NK cell activation, inferred from degranulation markers and cytokine production, was analyzed. Cell lines (MOLM-13 and MV-4-11) with CD155 and CD112 KO were generated to analyze the association between enhanced cytotoxic activity by DNAM-1 and nectin-related pathways. The anti-tumor effects of modified NK-92 cells were analyzed using in vivo imaging systems (IVIS) in a xenograft model with leukemic cell lines.
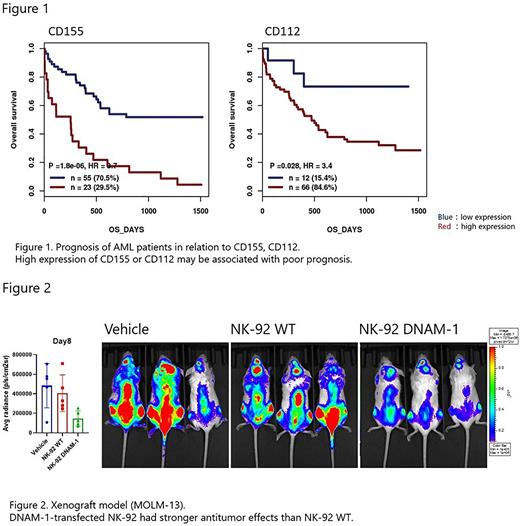
RESULTS: GenomicScape analysis showed that high expression of CD155 and CD112 is associated with shorter survival among AML cases (Figure 1). All myeloid leukemia cell lines expressed CD155 and CD112. WT NK-92 cells expressed TIGIT but not DNAM-1. Expressions of CD155 and CD112 were also confirmed in primary leukemic cells from de novo AML cases. Patients who did not achieve remission after initial therapy showed a trend toward a decreased DNAM/TIGIT ratio and high TIGIT expression on NK cells. Introduction of DNAM-1 or KO of TIGIT enhanced the cytotoxic activity of NK-92 cells by a killing assay using FACS. DNAM-1 transfection enhanced the expression of cytotoxicity-related genes, including granzyme B and granulysin. In addition, expression of cell killing-related genes, including interferon gamma (IFNγ) response and tumor necrosis factor alfa (TNFα) signaling, was also enhanced, whereas no similar changes were observed in NK-92 TIGIT KO cells. NK-92 DNAM-1 cells showed significantly increased expression of degranulation marker (CD107a) and intracellular cytokines (IFNγ and TNFα) when co-cultured with tumor cells. KO of CD155 and CD112 in tumor cells cancelled the enhancement of cytotoxic activity by NK-92 DNAM-1 cells, and TIGIT KO of NK-92 DNAM-1 cells did not further enhance cytotoxic activity. In a xenograft model, we confirmed the enhanced antitumor effect of NK-92 DNAM-1 cells compared with WT NK-92 cell by IVIS (Figure 2).
CONCLUSION: NK-92 DNAM-1 cells show enhanced anti-AML activity compared with WT NK-92 cells. High expression of DNAM-1 may overcome inhibitory activity by TIGIT. Modification of the DNAM-1/TIGIT/CD155/CD112 axis in NK cells may constitute an effective novel immune therapy for AML. Expression level of this axis may have a role as a prognostic marker in AML.
Disclosures
Nakamura:SymBio Pharmaceuticals: Speakers Bureau; AbbVie: Speakers Bureau. Nannya:Janssen Pharmaceutical: Speakers Bureau; Pfizer: Speakers Bureau; Takeda Pharmaceutical Company: Speakers Bureau; Astrazeneca: Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sumitomo Pharma: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Nippon Shinyaku: Speakers Bureau; Asahi Kasei Pharma: Speakers Bureau; Kyowa-Kirin: Speakers Bureau; Fuji Pharma: Honoraria; Filgen: Speakers Bureau; Otsuka Pharmaceutical: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Daiichi Sankyo RD Novare: Research Funding; Daiichi Sankyo Co., Ltd: Research Funding. Mitani:Janssen Pharmaceutical K.K.: Speakers Bureau; Sanofi K.K.: Speakers Bureau; SymBio Pharmaceuticals Ltd.: Speakers Bureau; Fujimoto Pharmaceutical Corp.: Speakers Bureau; Eisai Co., Ltd.: Speakers Bureau; Chugai Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Kyowa Kirin Co., Ltd.: Consultancy, Research Funding, Speakers Bureau; Nippon Shinyaku Co., Ltd.: Research Funding, Speakers Bureau; MSD K.K.: Speakers Bureau; Novartis Pharma K.K.: Consultancy, Speakers Bureau; Pfizer Japan Inc.: Speakers Bureau; Takeda Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Otsuka Pharmaceutical Co., Ltd.: Speakers Bureau; Daiichi Sankyo Co., Ltd.: Research Funding; AstraZeneca K.K.: Speakers Bureau; CSL Behring K.K.: Speakers Bureau; Amgen K.K.: Speakers Bureau; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding, Speakers Bureau; Bristol-Myers Squibb K.K.: Consultancy, Speakers Bureau; Alexion Pharmaceuticals, Inc.: Speakers Bureau; Ono Pharmaceutical Co., Ltd.: Speakers Bureau. Tamura:Janssen Pharma K.K: Other: Lecture fees; Sanofi K.K: Other: Lecture fees; Bristol Myers Squibb K.K: Other: Lecture fees; Ono Pharmaceutical Co: Other: Lecture fees. Imai:Janssen Pharmaceutical K.K: Speakers Bureau; Takeda Pharma: Research Funding, Speakers Bureau; Bristol Myers Squibb: Research Funding, Speakers Bureau; Sanofi: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.